Abstract
Introduction: Current treatment strategies for mature or peripheral T-cell lymphomas (PTCL) patients (pts) are largely unsatisfactory and outcomes for patients (pts) with rare gamma delta subtypes have not been well described. The purpose of the present analysis was to analyze clinical features and treatment outcomes for 99 pts with gamma delta subtypes (hepatosplenic T cell lymphoma [HSTCL], enteropathy T cell lymphoma [EATL ], and peripheral gamma delta T cell lymphoma [PGDT]) retrieved from an international prospective T cell lymphoma registry, the T-cell Project (TCP).
Methods: In this study, data were extracted from 1429 cases of newly diagnosed PTCL registered by 74 institutions world-wide in the T-cell Project and on whom baseline data, information on first line treatment, response to initial therapy, time to relapse and salvage treatment were available. For this analysis, 99 pts were identified who met the criteria of gamma delta subtype, including 24 with HSTCL, 62 with EATL, and 13 with PGDT. Central pathology review confirmed the diagnosis in all cases. Data for prognostic factors, initial and salvage therapies, and outcomes (Overall Survival [OS] and Progression-free Survival [PFS]) were extracted.
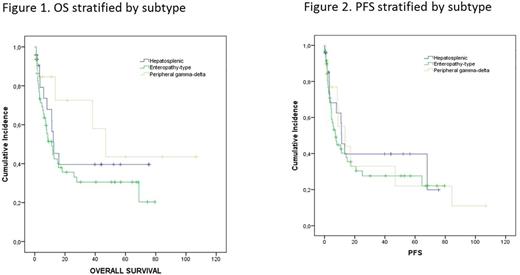
Results: Of the 99 cases identified, geographic distribution varied. Of EATL, 39 were from Europe, while HSTCL was slightly more frequent in the USA (44%) vs. Europe (16%). PGDT was found in USA and Europe, with no cases in South America and 2 cases in Asia. The median age for the 99 cases was 55 (range 18-86). The majority of patients were under age 60; prognostic factors included B symptoms (59%), advanced disease (69%), and monocyte count <800.mm3 (69%). Treatment records were locked and analysis was performed on 74 patients. Of those, front line therapy chosen by the treating physician consisted of chemotherapy alone (CT) in 53 (71%), CT followed by high dose therapy/stem cell rescue (HDT) in 9 (12%), and supportive care in 11. Anthracycline based CT was used in 56 (88%) of cases, but 6 (46%) of HSTCL patients received a non-anthracycline regimen. The overall response rate (ORR) was 45% with 20 (31%) CR (14 in CT alone patients and 6 in HDT patients). CR occurred in 40% of HSTCL, 30% of EATL, and 25% of PGDT. Forty-two percent of patients were refractory to front line therapy. Eleven pts underwent HDT in the salvage setting; of those only 3 pts with EATL had a CR, while one with EATL and one with PGDT had PR. At a median follow up of 51 months (95% CI 36-66), 47% of pts were alive, with a median OS of 13 months and median PFS of 11 months. There is no difference in OS or PFS at 3 or 5 yrs between EATL, HSCTL and PGDT (P=0.24 for OS, P=0.682 for PFS).
Conclusion: Because the gamma delta T cell lymphomas are rare and are under-represented in clinical trials, there is little data available describing treatment approaches and outcomes. In this cohort of 99 pts from the TCP registry, outcomes using the physician guided regimens are described and demonstrate that anthracycline based CT was the most common choice in first line therapy for EATL and PGDT, while non anthracycline based therapy is chosen for HSTCL. Overall CR rates were lower than those seen for nodal PTCL subtypes using similar treatment approaches. Surprisingly, despite NCCN guidelines suggesting that HDT is considered for consolidation in first remission, only a proportion of patients could undergo HDT, and HDT in the salvage setting had limited success. These results suggest that the incorporation of novel agents in the front line and the implementation of consolidation strategies, including stem cell transplantation and maintenance therapies should be considered for gamma delta T cell lymphoma subtypes.
Foss: celgene: Honoraria; immune design: Research Funding; spectrum: Honoraria, Speakers Bureau; Eisai: Honoraria; seattle genetics: Speakers Bureau. Horwitz: Infinity/Verastem: Consultancy, Research Funding; Mundipharma: Consultancy; ADCT Therapeutics: Research Funding; Kyowa-Hakka-Kirin: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Forty-Seven: Consultancy, Research Funding; HUYA: Consultancy; BMS: Consultancy; Aileron Therapeutics: Research Funding; Celgene: Consultancy, Research Funding; Millenium/Takeda: Consultancy, Research Funding. Kim: Mundipharma: Research Funding; Celltrion, Inc: Consultancy, Honoraria; Donga: Research Funding; J&J: Research Funding; Kyowa-Kirin: Research Funding; Takeda: Research Funding; Novartis: Research Funding; Roche: Research Funding. Hitz: Celgene: Consultancy. Gutierrez: JANSSEN: Consultancy, Research Funding, Speakers Bureau; ROCHE: Research Funding, Speakers Bureau; SERVIER: Speakers Bureau; GILEAD: Honoraria; TAKEDA: Speakers Bureau; PFIZER: Consultancy. Davies: Roche: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Other: Advisory Board, travel expenses to attend conference, Research Funding; Gilead: Consultancy, Honoraria, Other: Travel expenses to attend conference, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel expenses to attend conference, Research Funding; Bayer: Research Funding; Acerta Phamra: Research Funding; Karyopharma: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; GSK: Research Funding; CTI: Consultancy, Honoraria, Other: Travel expenses to attend conference; Seattle Genetics: Research Funding. Federico: takeda: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal